From microbial chaos to mucus loss, researchers reveal a disturbing new mechanism of microplastic-induced gut dysfunction in mammals.
As plastic pollution continues to invade every corner of our environment, its most invisible threat may lie within us. A groundbreaking study published in Nature Communications has uncovered a mechanistic pathway by which nanoplastics — the tiniest fragments of plastic — disrupt the gut microbiome and weaken intestinal barrier function.
Led by scientists from National Cheng Kung University in Taiwan, the research offers a stark warning: chronic exposure to nanoplastics can trigger a cascade of molecular dysfunctions, disrupting communication between host cells and beneficial gut microbes, and compromising the body’s frontline defense — the intestinal lining.
The Study: Tiny Plastics, Big Disruptions
In the study, mice were fed polystyrene nanoplastics — each approximately 100 nanometers wide — over a 12-week period. These nanoplastics are thousands of times smaller than a human hair and can permeate biological membranes more easily than larger microplastics.
After sustained exposure, the researchers found multiple layers of biological disturbance:
1. Barrier Breakdown
Two key proteins — occludin and claudin-1 — essential for maintaining tight junctions between gut epithelial cells, were significantly reduced. This degradation compromises the integrity of the intestinal wall, leading to a condition often referred to as “leaky gut”, where pathogens and toxins can pass into the bloodstream unchecked.
2. Microbial Imbalance
- Beneficial bacteria such as Lactobacillus were sharply reduced.
- Harmful or opportunistic bacteria like Ruminococcaceae increased in abundance.
- Uniquely, Lachnospiraceae was observed ingesting the nanoplastics, an unexpected interaction that altered how these bacteria secreted extracellular vesicles (EVs).
3. Disrupted Communication
The ingested nanoplastics triggered abnormal production of microRNA-containing extracellular vesicles, which are used by gut microbes to communicate with host cells. This disruption in EV signaling inhibited intestinal mucus production — a critical layer that protects the epithelium and nourishes healthy bacteria.
“This study is the first to show that plastic particles can interfere with the microRNA carried by extracellular vesicles between mouse intestinal cells and specific gut microbes, disrupting host–microbe communication,” said microbiologist Wei-Hsuan Hsu, the study’s lead author.
Microscopic images from tissue samples revealed a visible thinning of intestinal mucus, a condition associated with increased susceptibility to infection, inflammation, and chronic gastrointestinal disorders.
What Makes This Study Different?
While previous research has shown that microplastics reduce microbial diversity, promote inflammation, and damage gut barrier proteins, the Taiwanese study goes a step further: it identifies how plastic particles manipulate communication between gut bacteria and host tissues at the genetic and molecular level.
It connects nanoplastic exposure to:
- Disruption of microRNA (miRNA) signaling pathways
- Alteration in bacterial extracellular vesicle composition
- Suppression of mucin gene expression, reducing mucus production
- Changes in bacterial gene activity and protein synthesis
Together, these changes reflect a systemic breakdown of the gut’s regulatory systems, increasing the risk of chronic diseases, immune dysregulation, and even neuroinflammation.
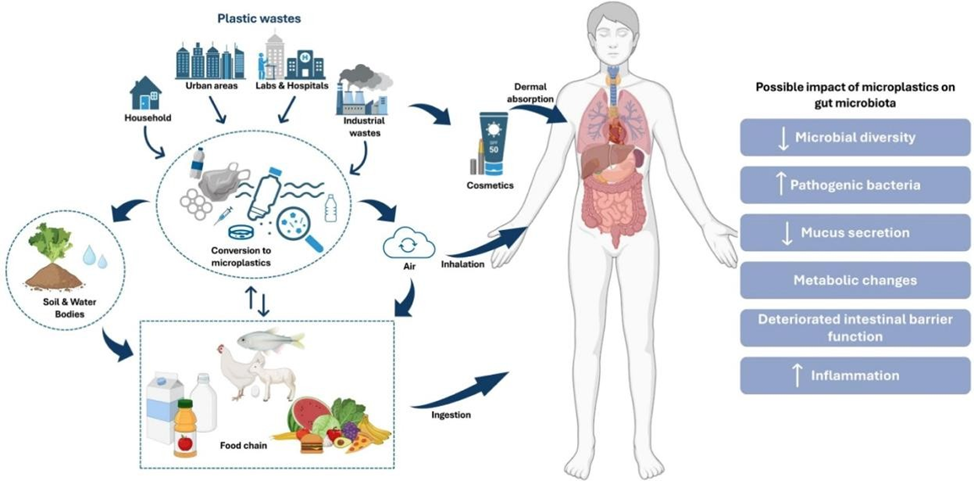
Implications for Human Health
Although mice are not humans, they share many fundamental biological pathways with us — especially in gut physiology and microbial communication. Researchers caution that humans are not yet known to be exposed to such high levels of nanoplastics. However, the mechanisms revealed here are highly relevant to ongoing concerns about long-term, low-dose microplastic ingestion in people.
“Given the current limitations in nanoplastic detection technologies and the uncertainties associated with extrapolating animal model results to humans, continued research is critical,” said immunologist Yueh-Hsia Luo, from National Central University, who was not involved in the study.
Additional research has shown that nanoplastics may breach the intestinal barrier and accumulate in distant organs including the liver, kidneys, lungs, brain, and lymph nodes. The plastic particles often carry toxic additives such as BPA, phthalates, and polycyclic aromatic hydrocarbons (PAHs), which are linked to hormonal disruption, immune dysfunction, cardiovascular damage, and increased cancer risk.
What Can You Do?
While the science evolves, experts agree there are steps individuals can take now:
- Filter your drinking water: Studies suggest switching from bottled to filtered tap water can reduce plastic intake by up to 90%.
- Reduce plastic use: Avoid heating food in plastic, cut down on packaged goods, and choose glass or stainless steel when possible.
- Support your gut: A fiber-rich diet with fruits, vegetables, and fermented foods (like yogurt and kimchi) can help restore microbial balance.
- Consider probiotics: Research in animal models shows Lactobacillus and Bifidobacterium strains may repair gut lining and aid in detoxifying plastics.
- Push for policy change: Governments must act on regulating plastic production, improving detection methods, and funding human studies.
Conclusion: Plastic’s Hidden Pathways
This study makes one thing clear: nanoplastics are not passive environmental bystanders. They are bioactive particles that alter communication networks within our bodies, disrupt microbial partnerships, and weaken our biological defenses from the inside out.
As plastics continue to infiltrate our food, water, and air, the urgency for comprehensive exposure assessments and clinical research in humans grows.
We may not feel it yet — but at a microscopic level, our guts are already under siege.
References:
Mixture Effects of Polystyrene Microplastics on the Gut Microbiota in C57BL/6 MiceBei Gao, Xiaochun Shi, Meng Zhao, Fangfang Ren, Weichen Xu, Nan Gao, Jinjun Shan, and Weishou ShenACS Omega 2025 10 (8), 7597-7608DOI: 10.1021/acsomega.4c00645